Give me 3 - Reversible changes
Science Resource Description
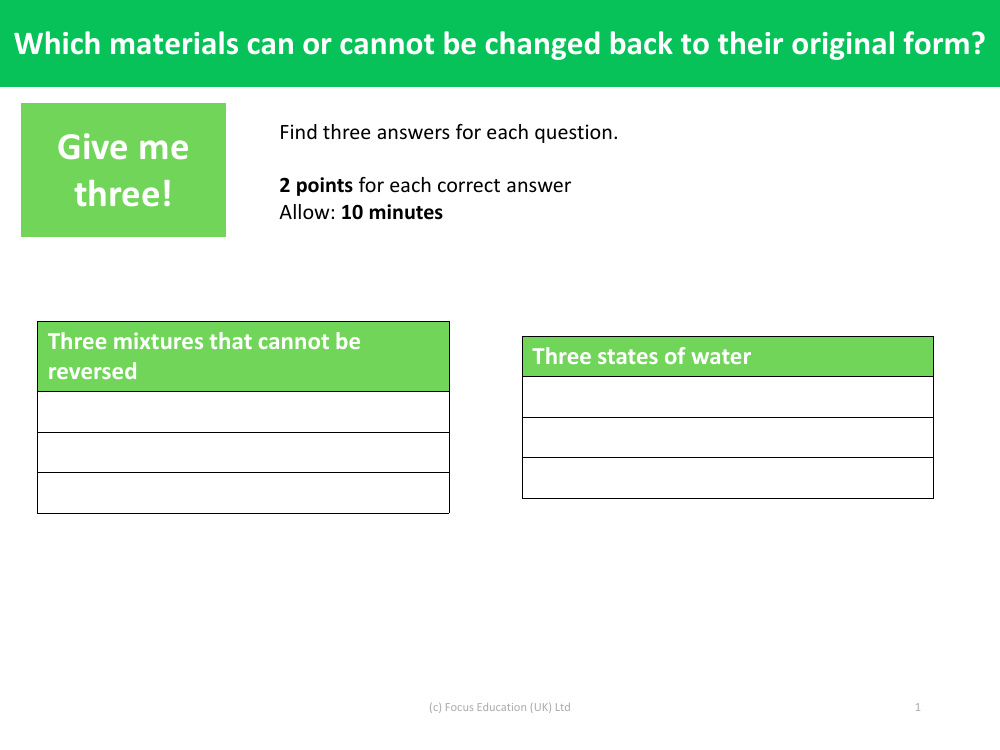
Reversible changes refer to physical changes in materials that can be reversed, returning the substances to their original state. For the first question, three states of water that exemplify reversible changes are ice, liquid water, and water vapour. Ice can melt to become liquid water and can be refrozen to become ice again. Similarly, liquid water can be heated to become water vapour in the form of steam, and when cooled, it condenses back into liquid water. These transformations between solid, liquid, and gas phases of water are classic examples of reversible changes.
On the other hand, some mixtures undergo changes that cannot be easily reversed. An example of such an irreversible mixture would be concrete, which once set, cannot be returned to its original components of cement, water, and aggregate. Another example is baking a cake; once the ingredients are mixed and baked, they undergo a chemical reaction that cannot be undone. A third example is the mixing of an acid and a base to form water and a salt; this neutralisation reaction produces new substances that are not easily separated into their original components. These instances illustrate mixtures and reactions where the original materials cannot be changed back to their original form, highlighting the concept of irreversible changes.