Dissolving, Mixtures and Changes of State - Results Tables
Science Resource Description
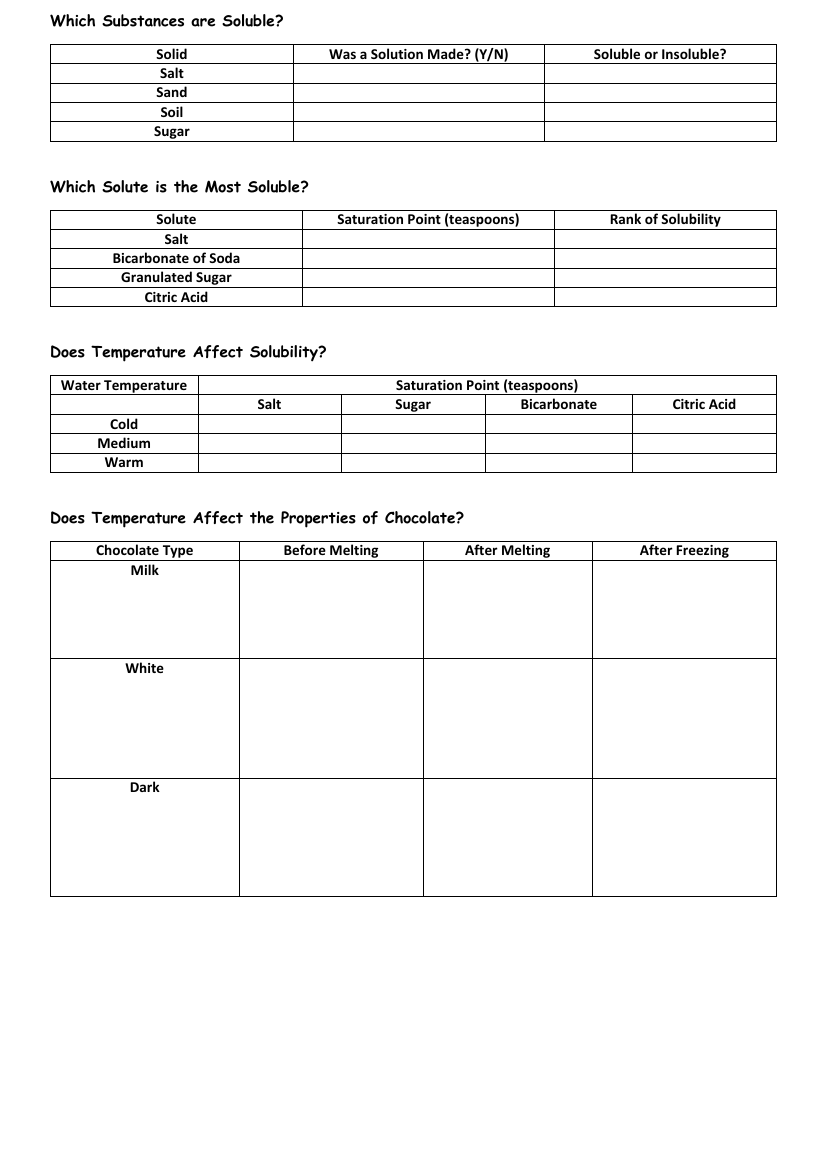
The exploration of solubility begins by distinguishing which substances can dissolve in a solvent to form a solution. Students investigate the solubility of various solids such as salt, sand, soil, and sugar, recording whether a solution was made and classifying each solid as soluble or insoluble. This forms the basis for understanding the nature of different materials and their interaction with solvents. The next step in the investigation involves determining which solute has the highest solubility. Using a saturation point measured in teaspoons, students rank solutes including salt, bicarbonate of soda, granulated sugar, and citric acid, thus comparing their ability to dissolve at a given concentration.
Further inquiry delves into the effects of temperature on solubility. Students examine how the saturation point of different solutes—salt, sugar, bicarbonate, and citric acid—changes with water at cold, medium, and warm temperatures, thereby observing the influence of thermal energy on the dissolving process. Additionally, the investigation explores how temperature affects the properties of chocolate. By observing milk, white, and dark chocolate before and after melting, as well as after freezing, students gain insights into the physical changes and the stability of chocolate when subjected to temperature variations. These practical experiments provide a hands-on understanding of solubility, mixtures, and changes of state.





