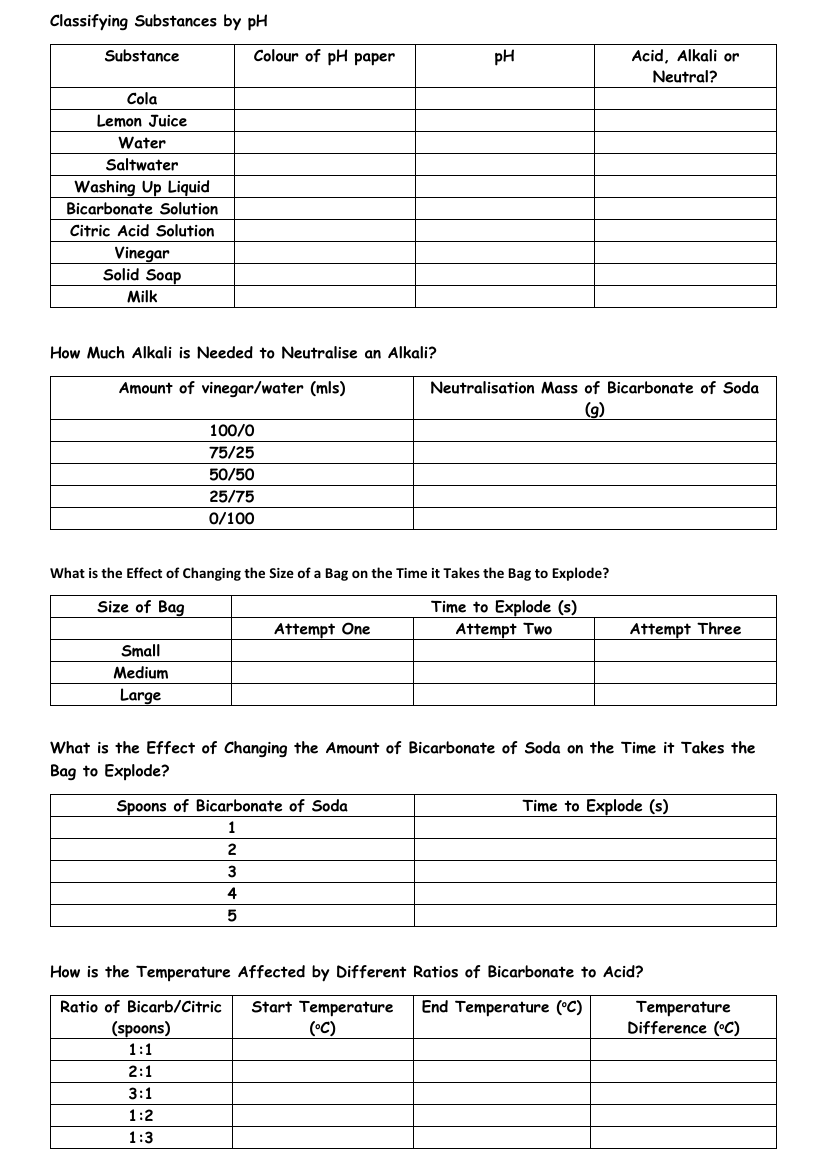
Acid and Bicarbonate of Soda - Results Tables
Science Resource Description
The provided text outlines various scientific experiments and observations involving the classification of substances by their pH levels, the neutralisation of acids with alkalis, and the effects of changing variables on reaction times and temperatures. The first section details a series of substances, including cola, lemon juice, and vinegar, which are tested with pH paper to determine their pH levels and classify them as either acidic, alkaline, or neutral. This classification helps students understand the chemical nature of everyday substances.
Subsequent sections describe experiments that explore the interactions between acids and bicarbonate of soda (an alkali). One experiment measures the amount of bicarbonate of soda required to neutralise different vinegar/water mixtures. Another investigates the impact of bag size on the time it takes for a reaction-induced explosion when bicarbonate of soda is used. Additionally, the time to explosion is also measured by varying the amounts of bicarbonate of soda added. The final experiment examines how different ratios of bicarbonate of soda to citric acid affect the temperature change of the reaction, with the results recorded as starting and ending temperatures, as well as the temperature difference. These activities are designed to provide hands-on learning experiences about chemical reactions and their measurable effects.





