Acid and Bicarbonate of Soda




Science Lesson Description
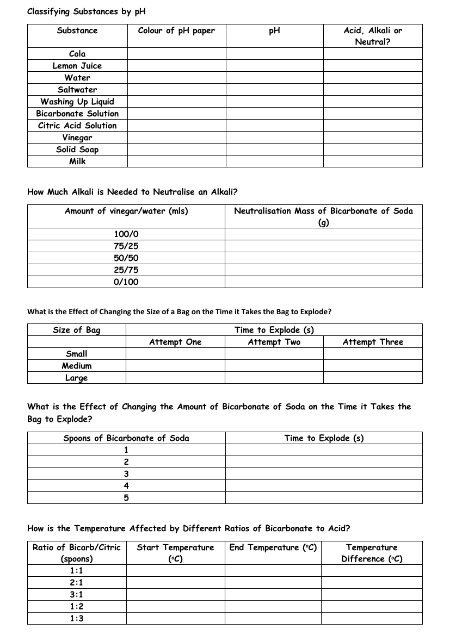
The main focus of this lesson is to explain that some changes result in the formation of new materials, which is not usually reversible. Students will learn about chemical reactions and the signs that indicate a reaction has occurred, such as the formation of gas, heat, or light. They will also learn to identify the products of chemical reactions. The class will also classify substances as acids, alkalis, or neutral based on their pH level, which is a measure of the ability of a substance to produce hydrogen in solution. The pH scale ranges from 1-14, with values below 7 being acidic, values above 7 being alkaline, and 7 being neutral.
This lesson is aligned with the previous learning in Kindergarten to identify and name everyday materials and in 1st Grade to find out how solid objects' shapes can be changed. The activity will involve using pH paper and a variety of substances such as cola, lemon juice, bicarbonate of soda, vinegar, and more to classify the substances as acid, alkali, or neutral. Students will work in groups of four and follow the steps of tearing off some of the pH paper and dipping it into the sample, observing the color change, and making a judgement of the pH level.
In the second activity, students will learn about neutralizing acids. They will measure out different amounts of vinegar and bicarbonate of soda, stir the mixture, and test the pH level until the paper turns green. They will repeat the process with different ratios of vinegar and bicarbonate of soda to understand the effect of adding more or less of either substance on the pH level.
Cross-Curricular Links
- DT: Research the role of acids in etching
- math: Produce graphs of experimental results
- History: Write an essay on the role of acids in the first battery made
Important Lesson Guidance
Before the lesson, the teacher will need to obtain some cola, bicarbonate of soda, and lemon juice. For the pH classification activity, the teacher has free reign on the substances tested, as long as they are safe for students to handle. The solid samples will need to be mixed with a small amount of water for testing. In the neutralizing acids activity, the equipment required includes clear plastic cups, vinegar, bicarbonate of soda, pH paper, teaspoons, and a measuring cylinder. The students will work in groups of two.
Influential Scientists
Soren Sorensen, Jons Berzelius, Humphrey Davey.
Keywords
Irreversible, Acid, Alkali, pH scale, Neutral, Carbon Dioxide, Neutralization.



















